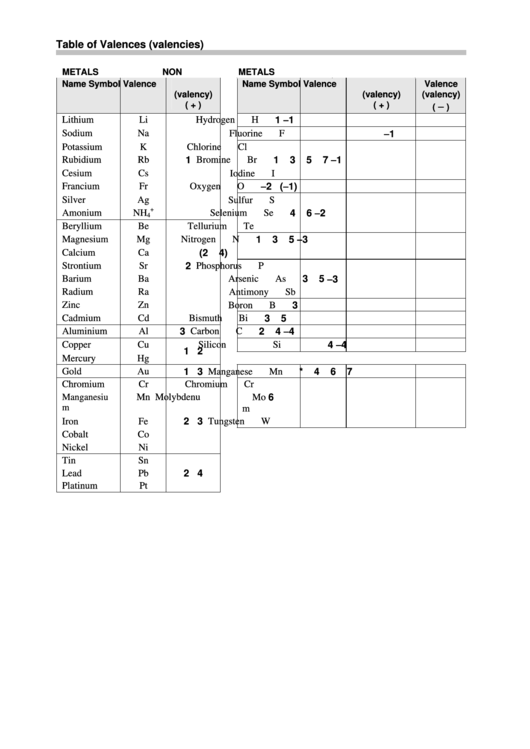
Chemical Table Of Valences (Valencies) printable pdf download
The electrons present in the outermost shell of an atom are referred to as valence electrons. The count of these valence electrons determines the valency of an atom. For elements in the s-block and p-block of the periodic table, valency is calculated as the number of valence electrons or eight minus the number of valence electrons.

valency table Scribd india
Valency is a fundamental concept in chemistry that describes an element's ability to combine with other elements and form compounds. The Valency For All the Elements determines the number of electrons it gains, loses, or shares when forming chemical bonds. This paragraph will provide an overview of the valency for all the elements in the periodic table, highlighting the diversity of chemical.
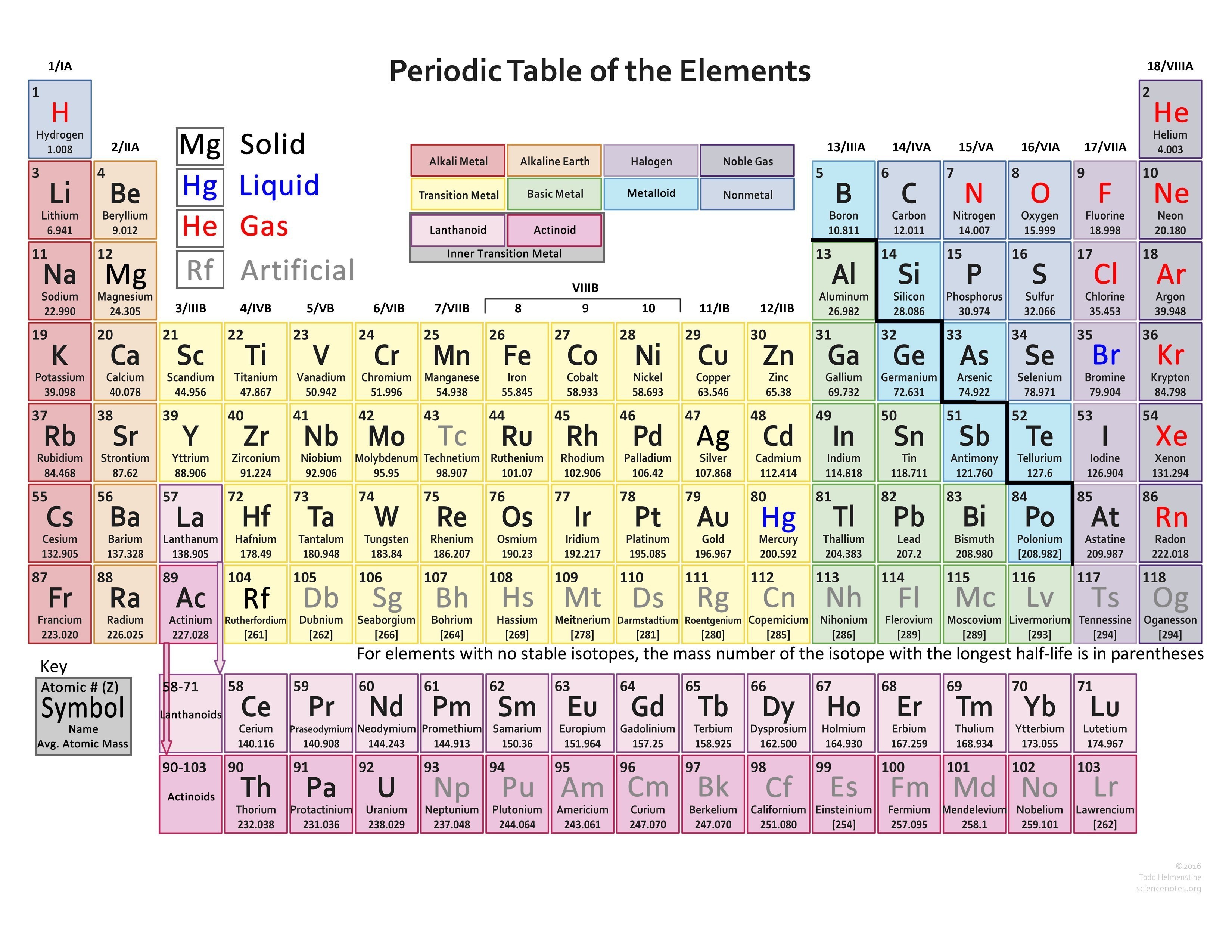
Find Various Types of Valency of Elements Valencies of 118 Elements
Easy way to understand the Valency Chart, calculation of valency by formula. Students of class 7th & 8th can understand in a short time how to calculate Vale.
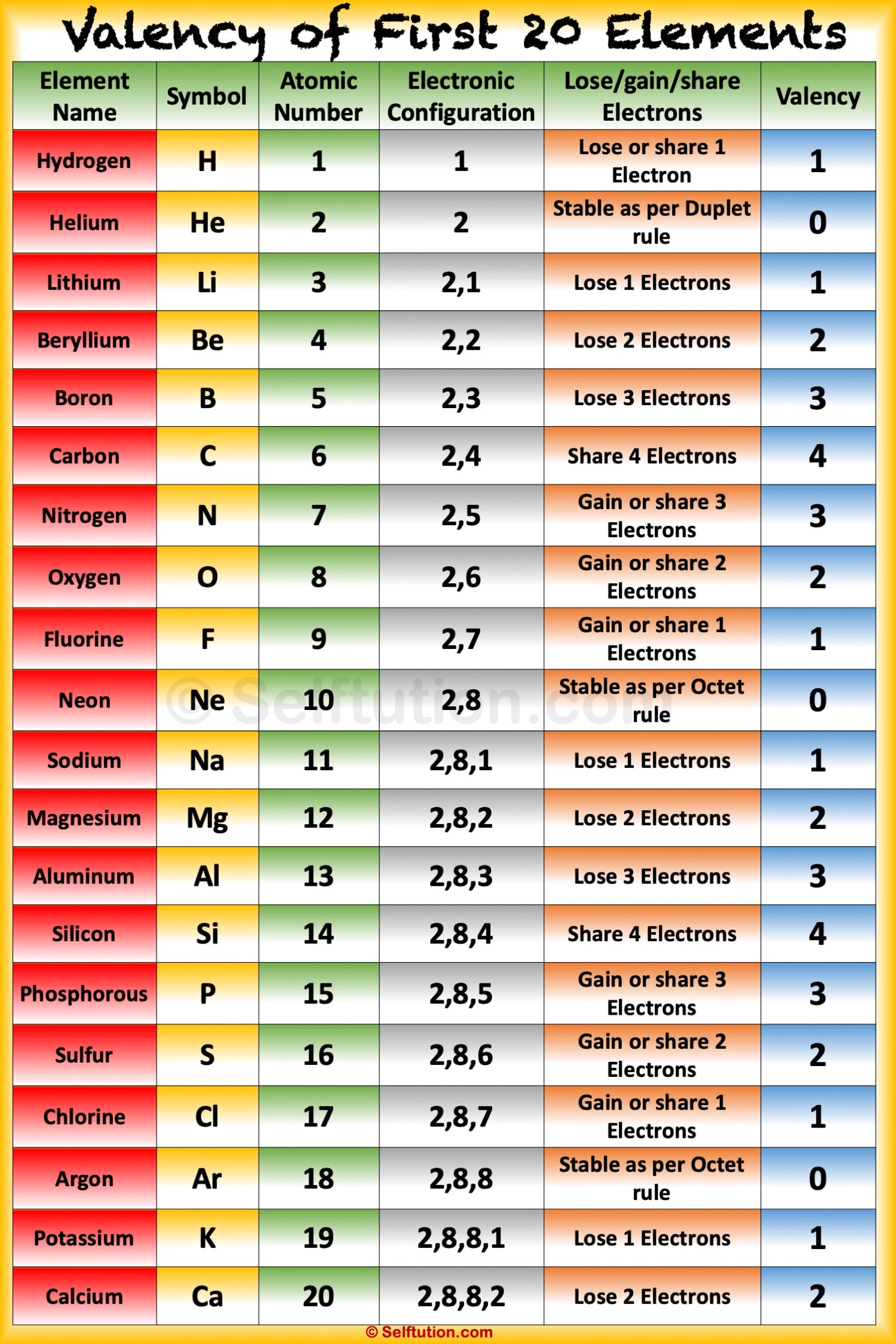
Valency and Variable Valency Valence Shell and Electrons » Selftution
Valency is the combining capacity of an element. It is always a whole number. It has no plus or minus sign. The electrons present in the outermost shell of an atom are known as 'Valence Electrons'. We can say valency is the number of electrons an element can lose or gain to attain stability.

printable periodic table with valence charges printable periodic table with valence charges
Valency therefore provides teachers with a simple way of introducing the Periodic Table. A major problem with using atomic structure is that students have to take so much on trust. 8 . Chemists use valency in nomenclature. For Main Group atoms that exhibit variable valency, they choose a standard value and cite non-standard ones, using the.

Valency Chart Chemistry, Basic facts, Chart
A Valency Chart is intended for determining the amount of chemical bonds that a specific element can make with different elements. The valency table is a list of the element's valencies. Based on the element's valence electrons, those are the outermost electrons in charge of bonding, it describes how an element can form bonds.

Valency Table Chemical Compounds Periodic Table
The number of valence electrons determines most of an atom's chemical behaviors. So, it's important to be able to identify how many valence electrons atoms of different elements have. Fortunately, we can use the periodic table to quickly determine the number of valence electrons for main group elements. Valence electrons on the periodic table

Periodic trend of valency Chemistry Khan Academy YouTube
Valence, in chemistry, the property of an element that determines the number of other atoms with which an atom of the element can combine. Introduced in 1868, the term is used to express both the power of combination of an element in general and the numerical value of the power of combination. A.

Valence Electrons Definition, Examples, Chart, Periodic Table
Chemistry Structure of Atom Valency What Is Valency? Define Valency The combining capacity of an atom is known as its valency. The number of bonds that an atom can form as part of a compound is expressed by the valency of the element. We all know how electrons in an atom are arranged in shells/orbitals.
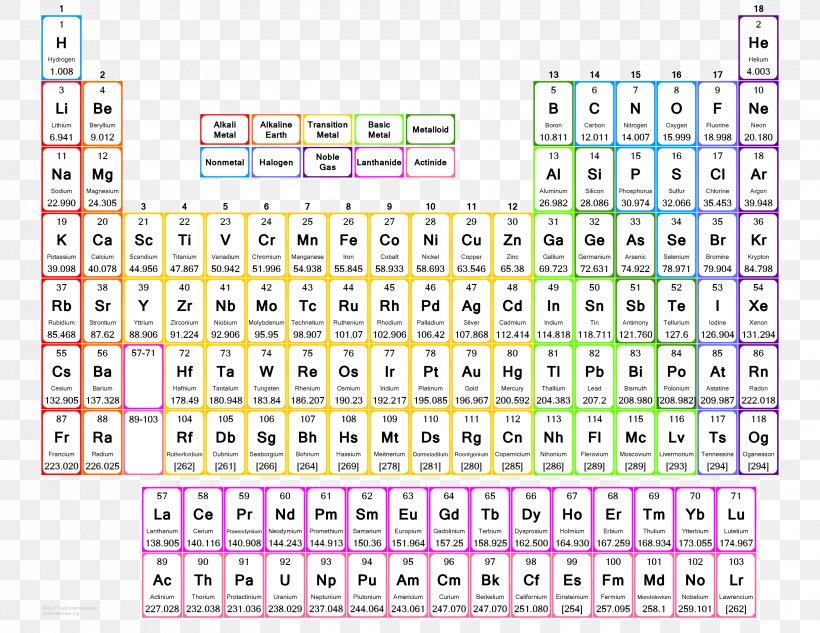
Periodic Table Of Elements With Atomic Mass And Valency Bruin Blog
The valency of an element is a measure of its combining capacity and can be defined as the number of electrons that must be lost or gained by an atom to obtain a stable electron configuration. What does the term 'Oxidation State' mean? The oxidation state of an atom is the number of electrons lost or gained by it.
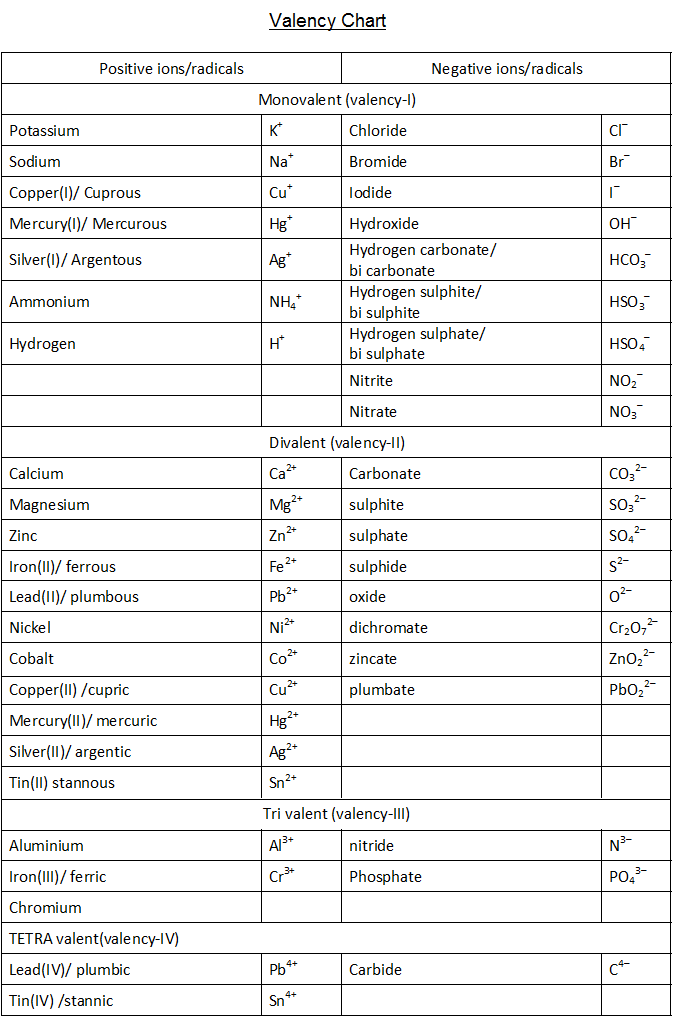
VALENCY CHART
Valence is the number of connections an atom tends to form. H is defined to have a valence of 1. For instance: methane, CH 4: carbon atoms have a valence of 4. water, H 2 O: oxygen has a valence of 2. lithium oxide, Li 2 O: lithium has a valence of 1. hydrogen sulfide, H 2 S: sulfur has a valence of 2. aluminum oxide, Al 2 O 3: aluminum has a.

Free Printable Periodic Table (With names, charges & Valence Electrons) [PDF] Printables Hub
Hence we assign a valence of 1 to H and to Cl. The valence of O is twice as great, and so we assign a value of 2. Example 4.4.1 4.4. 1: Formula Predictions. Use the data in the first table to predict what formula would be expected for a compound containing (a) sodium and fluorine; (b) calcium and fluorine.

Elements Their Atomic, Mass Number,Valency And Electronic Configuratio How to tell how many
2) Using the Periodic Table. In this method, valency is calculated by referring to the periodic table chart. For example, all the metals, be it hydrogen, lithium, sodium and so on, present in column 1 have valency +1. Similarly, all the elements present in column 17 have valency -1 such as fluorine, chlorine, and so on.
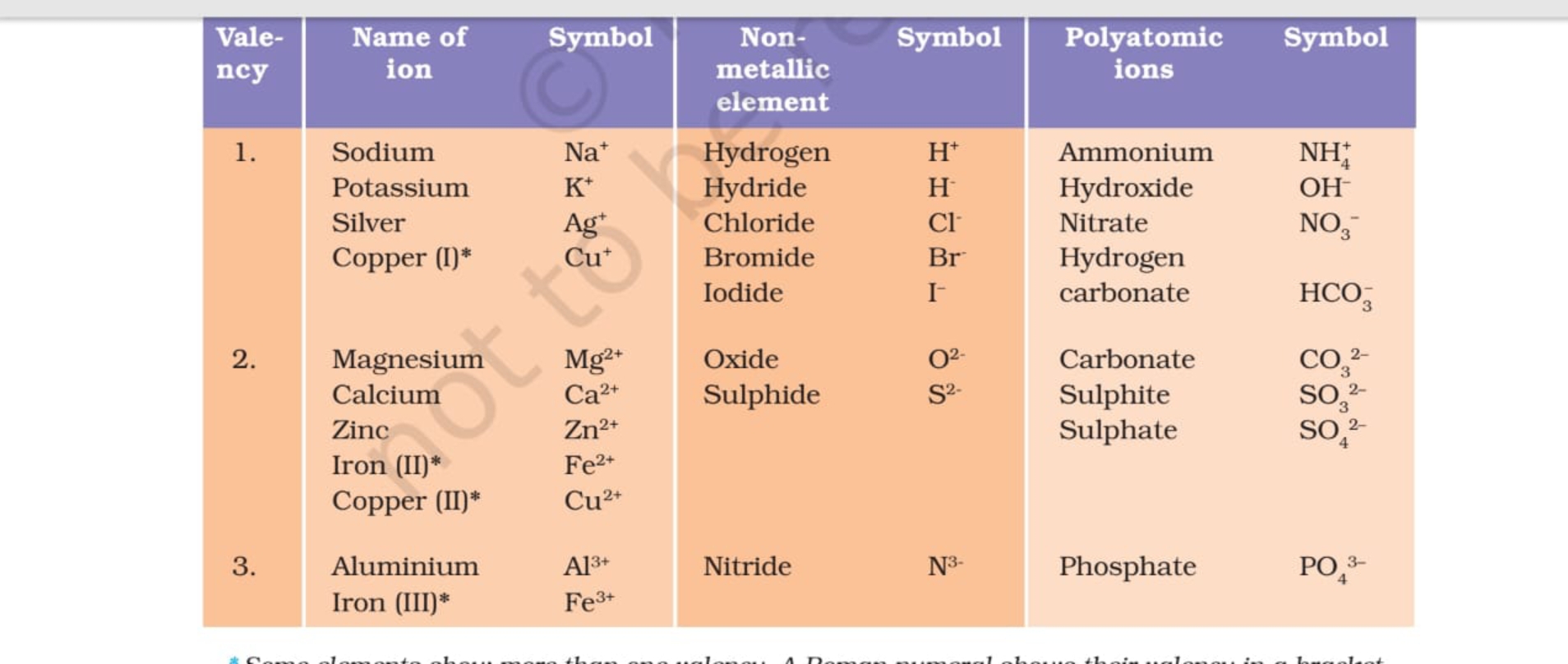
Valency Table Science Notes Teachmint
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemical bonds that each atom of a given element typically forms.

Valency Chart
You may assume that the valences of the elements—the number of electrons with which an atom will bond or form—are those that can be derived by looking at the groups (columns) of the periodic table. While these are the most common valences, the real behavior of electrons is less simple. Here is a table of element valences.

ICSE Class 9 Chemistry Additional Charts Reference Valency Chart PDF
Valence is a measure of how readily an atom or radical forms a bond. Jason Reed / Getty Images By Anne Marie Helmenstine, Ph.D. Updated on September 30, 2018 The words valence and valency have two related meanings in chemistry. Valence describes how easily an atom or radical can combine with other chemical species.